CAQ software for production & logistics
CAQ system & predictive quality: proactive management of product & process
For industrial companies, predictive quality is the ideal way to lower quality costs, more stable processes, more reliable products and higher customer satisfaction. Our integrated CAQ Germanedge provides the necessary transparency along the entire process chain.
The SaaS-capable Software brings together the development, production and logistics data relevant to quality management on a central platform. The permanent, fully automatic data comparison allows you to find out at the earliest possible stage whether and which parameters are at risk of falling out of specification. Machine learning algorithms help you to continuously increase the scope of your analytics.
Advantages of our CAQ software

Modules of our CAQ system
Computer Aided Quality Assurance (CAQ) encompasses many aspects that influence each other – and must be considered simultaneously. These include, for example
- Failure Mode and Effects Analysis (FMEA): This plays a key role in risk assessment and mitigation. This in turn is essential for quality planning.
- Supplier evaluation: This ensures that only materials of the highest quality are used in the production process.
- Action management: This enables companies to react quickly to quality fluctuations or problems and initiate measures.
- In sectors such as medical technology – where strict quality standards apply – effective quality management is very important. Methodical approaches are key to continuously improving quality.
- Cooperation with partners – including suppliers – is essential to achieve and maintain quality objectives.
- Production processes must meet quality-related requirements and fulfill customer expectations.
Risk minimization right from the start
Our APQP Software software unlocks the benefits of predictive quality assurance during development. It allows your engineers to identify defects before production ramps up, giving you effective quality management. At its core, APQP is a project management solution that enables engineering to utilize existing QM along the entire value chain in its development projects. The preventive approach minimizes the risks of rejects and rework, which are particularly high in the case of new launches or process adaptations.
Fully automated data integration for end-to-end workflows
The measurement data management software in our CAQ suite lays the data foundation for your predictive quality approach. Fully automated, Germanedge CAQ merges the various inspection data in a central database, where they are processed further in the context of the order data. Our measurement data management has several hundred interfaces as well as additional integration technologies (e.g. event bus).
This allows you to connect measuring machines of any type at minimal cost. Flexibly configurable analysis tools are available for evaluation and reporting. In the event of deviations, measurement data management synchronizes with the other modules of the CAQ software so that suitable countermeasures can be initiated fully automatically. This creates consistent workflows for the standard-compliant and cost-optimized elimination of problems.
Integrated service management in testing technology
The measurement and test equipment management of our Software helps you to get the most out of your measuring and testing technology. The software allows your service teams to plan all maintenance measures with foresight, carry them out economically and document them in an audit-proof manner. This also benefits your colleagues in the value chain – whether in the incoming goods warehouse, laboratory, production or dispatch. This is because each area can always assume that it has correctly calibrated measuring equipment at its disposal to verify the quality of the products and detect defects reliably and quickly.
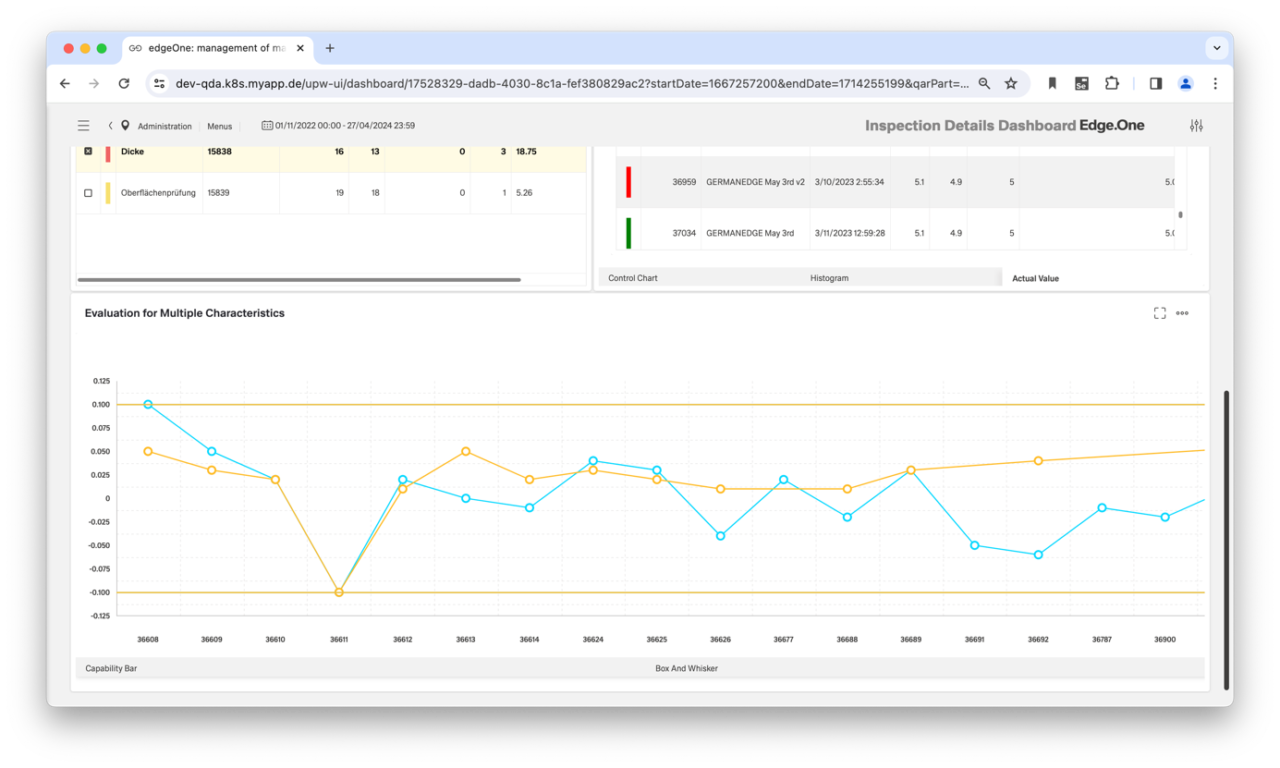
Optimum process and machine capability
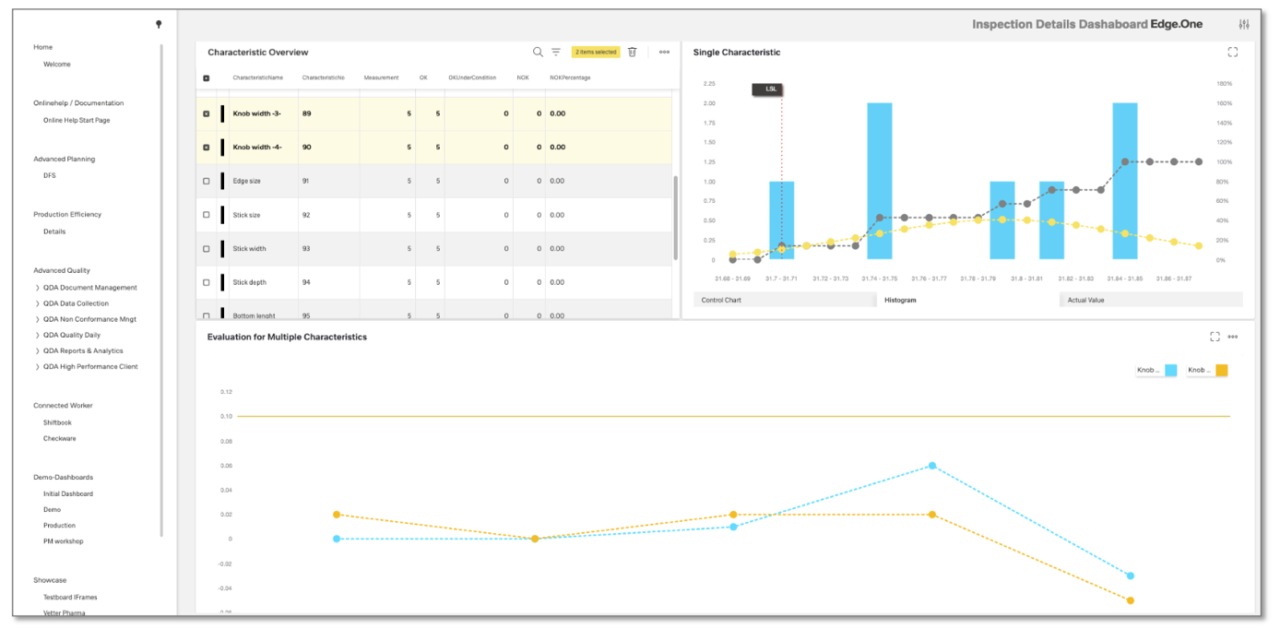
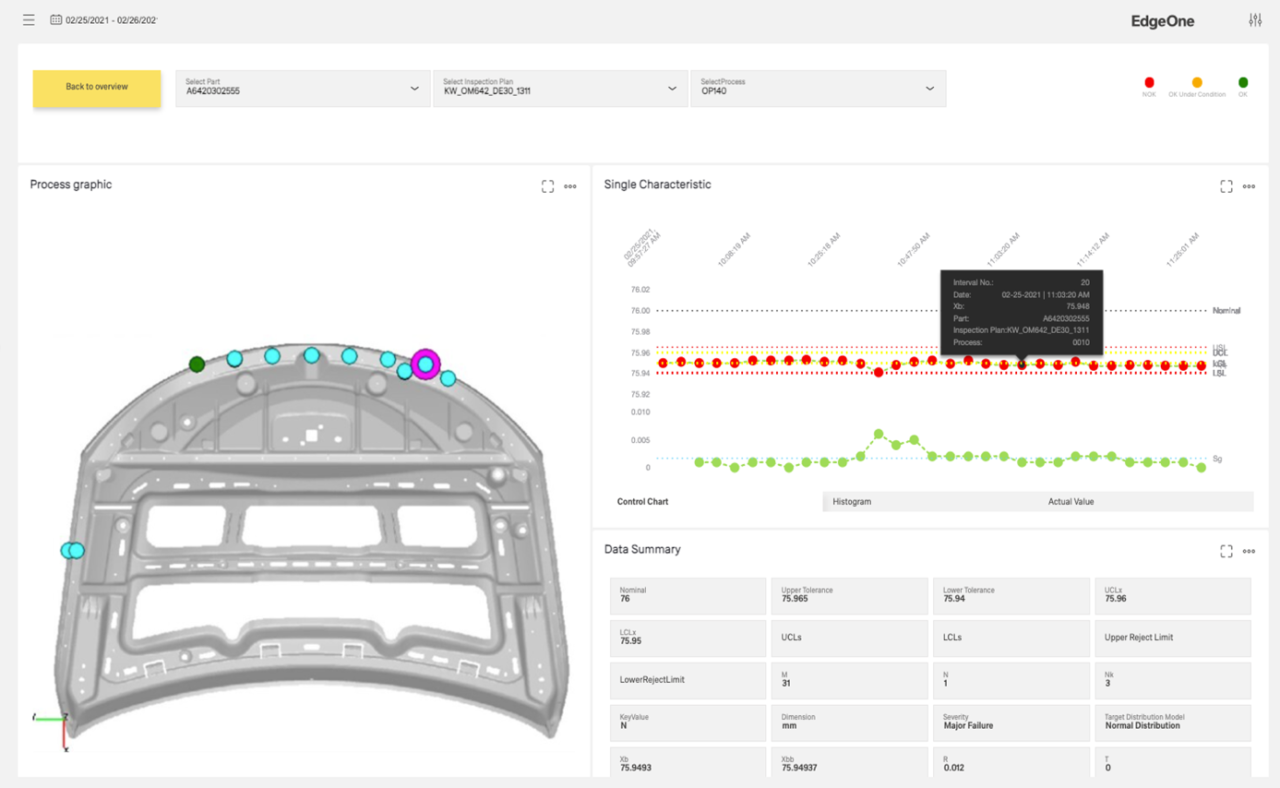
Thanks to its open system architecture, the SPC software of our CAQ software can synchronize with testing devices and measuring machines of all kinds. All inspection data is recorded fully automatically and in real time. In addition, the data analysis (QDA) can be extended with suitable metadata such as tolerance limits. In this way, you can optimize both machine capability and process capability. Furthermore, the measured values can be enriched with context data (e.g. temperature curves) and evaluated using suitable machine learning algorithms. This provides your Corrective and Preventive Actions (CAPA) approach with new ideas for identifying error patterns and eliminating their causes.
Resolving bottlenecks in the laboratory
The LIMS (Laboratory Information and Management System)is an integral part of our CAQ. In a fundamental first step, the LIMS calculates the most economical sequence of the current inspection orders. To do this, the software compares itself with the order management of your ERP system. This means that the allocation of laboratory resources is based on the quantity structure of production planning. Building on this, we offer you detailed planning software with which you can make your resource management even more flexible. For example, in the event of short-term order fluctuations. As soon as the laboratory planning is complete, the LIMS controls and monitors the analysis orders and compliance with all relevant standard specifications. This ensures that all laboratory approvals are available on time and that the associated production batches can start on schedule.
Full information capability throughout the entire product life cycle
For product data traceability, we offer you the CAQ module of the traceability software. This allows you to reliably monitor and verify the quality of your products in all phases of the product life cycle. Possible field problems are identified at an early stage so that you can proactively maintain faulty products or take them back at low cost.
The increase in transparency also helps to strengthen customer loyalty. Because: Traceability QM gives you the information you need to be able to provide your customers with full information at all times. For example, when it comes to proving the environmental compatibility of the components and preliminary products used. The same applies to communication with your auditors, whose interest in inspections is also constantly increasing. As an integral part of our CAQ software, Traceability QM has access to all relevant product data. This gives you a comprehensive knowledge base to provide reliable answers to the questions of the various stakeholder groups.
100% conformity to standards and audit compliance
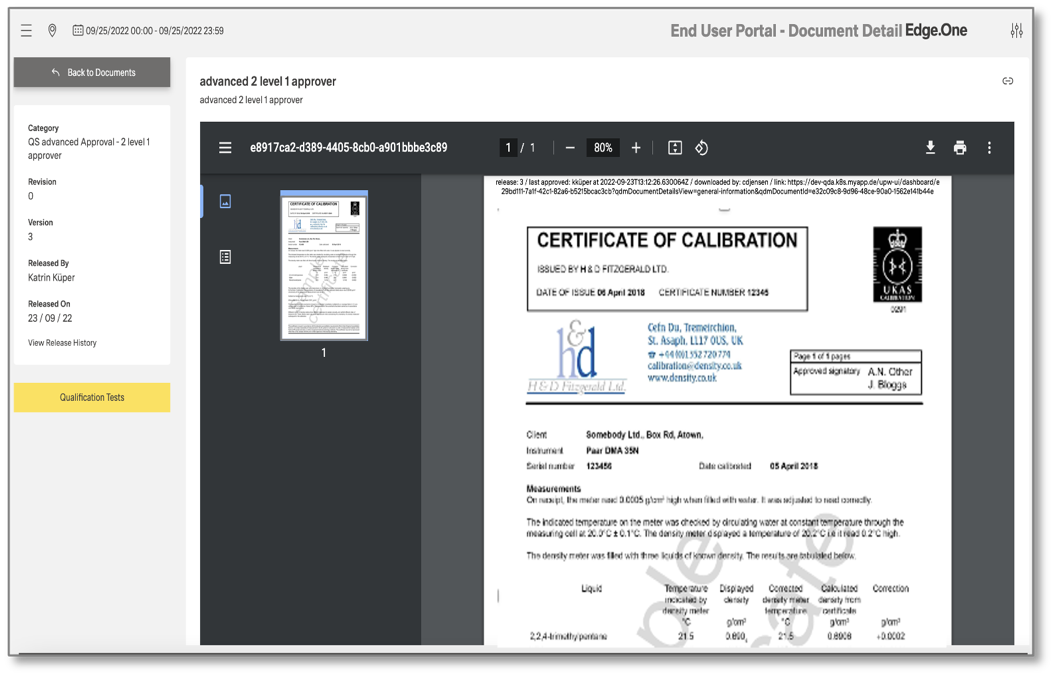
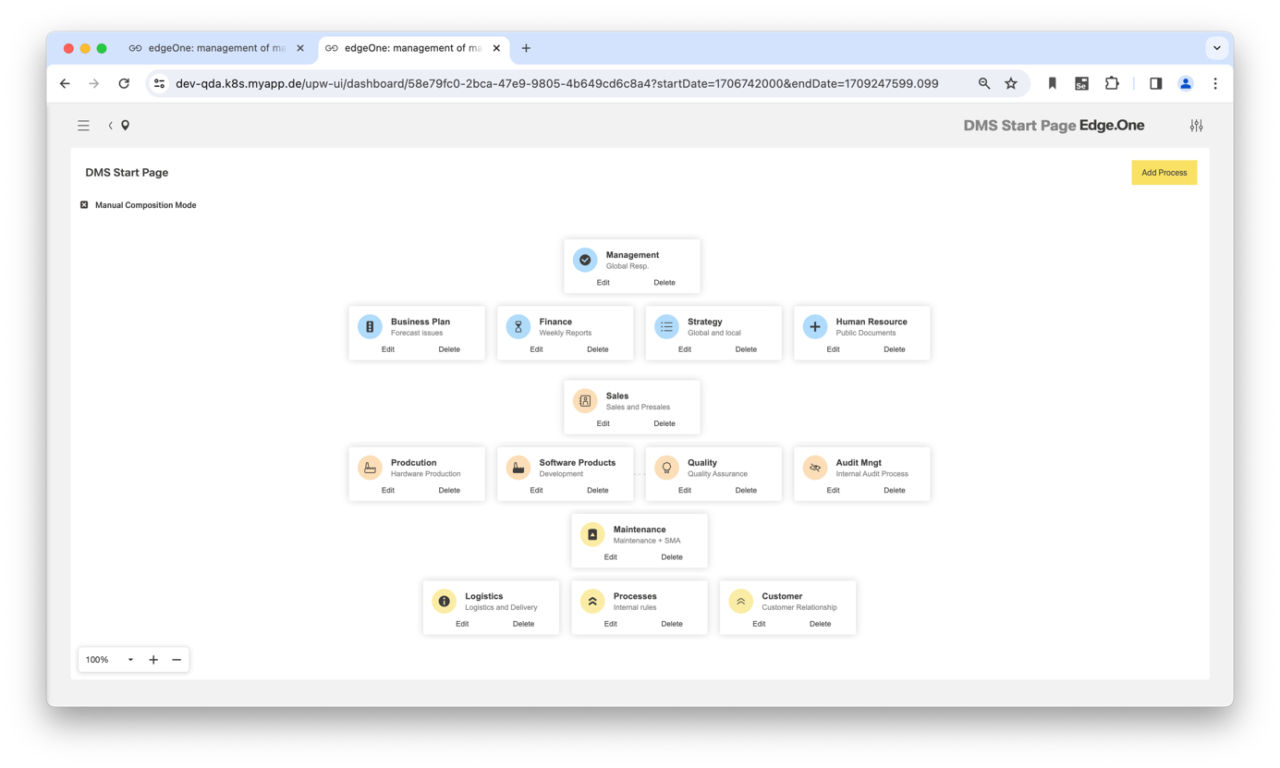
One of the success factors of a functioning preventive quality approach is maximum transparency. The document management system (DMS) of our CAQ software plays a central role in this and supports compliance with the ISO 9001:2015 and ISO 15489 standards, for example. The DMS controls and monitors the entire life cycle of your QM documents. You receive standard-compliant workflows for recording, approval, distribution and version control. As the DMS records all status changes in the audit trail, it provides unlimited audit security. The DMS therefore serves as a valid knowledge repository for the entire value chain. It can be accessed via a user-friendly browser app.
Learning from mistakes
In order to process internal and external complaints as quickly as possible, our CAQ system also has fully integrated complaint management software.
When complaints are received, the software brings together the appropriate players in a team in accordance with standards. The team members are provided with the information and tools they need to solve the problem at hand. Even multi-level complaint cases can be processed reliably and quickly. The integrated escalation management monitors compliance with the time and cost plans associated with the complaints. Completed complaints serve as a source of ideas for the continuous improvement process (CIP). Possible repeat errors can thus be systematically eliminated.
Integration of the CAQ software

Integrate the CAQ system step-by-step:
- Contact us
- We show you the solution in a live demo
- We discuss the requirements and technical integration with you
- We support you with the integration and onboarding

All options are open to you for using and operating the CAQ system: You have the choice between an:
- On-premise solution in your own data center
- Software-as-a-Service (SaaS) use in a private, hybrid or public cloud environment.
To achieve maximum scalability, availability and data security, we recommend using leading cloud hyperscalers such as Amazon, Azure, Google or Ionos. SaaS products run on our Edge.One platform.
Edge.One is the platform as a service solution for your CAQ processes too. The future of digital production does not consist of various isolated solutions and the resulting separate data streams and work areas. Instead, it will consist of an intelligent, integrated flow of all your data, resources and processes.
To break down existing silos and create an ecosystem for all MES and MOM processes, you need an intelligent data hub. The store floor must be connected to the business level, the supply chain to quality control management, complaints management to advance quality planning: this is the only way to sustainably increase production efficiency and generate the power of smart data from big data.
Our CAQ software can be seamlessly integrated into your entire system landscape. This enables the bidirectional exchange of all QM-relevant data along the entire value chain. The integration begins with the master and order data from your CAD, PDM, ERP and SCM systems and extends to the process data from machine control and robotics through to the measurement data from inspection technology.
In many places, the majority of QM data comes from suppliers. The open architecture of our CAQ system enables you to integrate suppliers in line with roles and standards, for example via lean portal solutions. The data is transmitted via specially secured Internet connections.
What is Computer-aided Quality assurance (CAQ)?
Definition
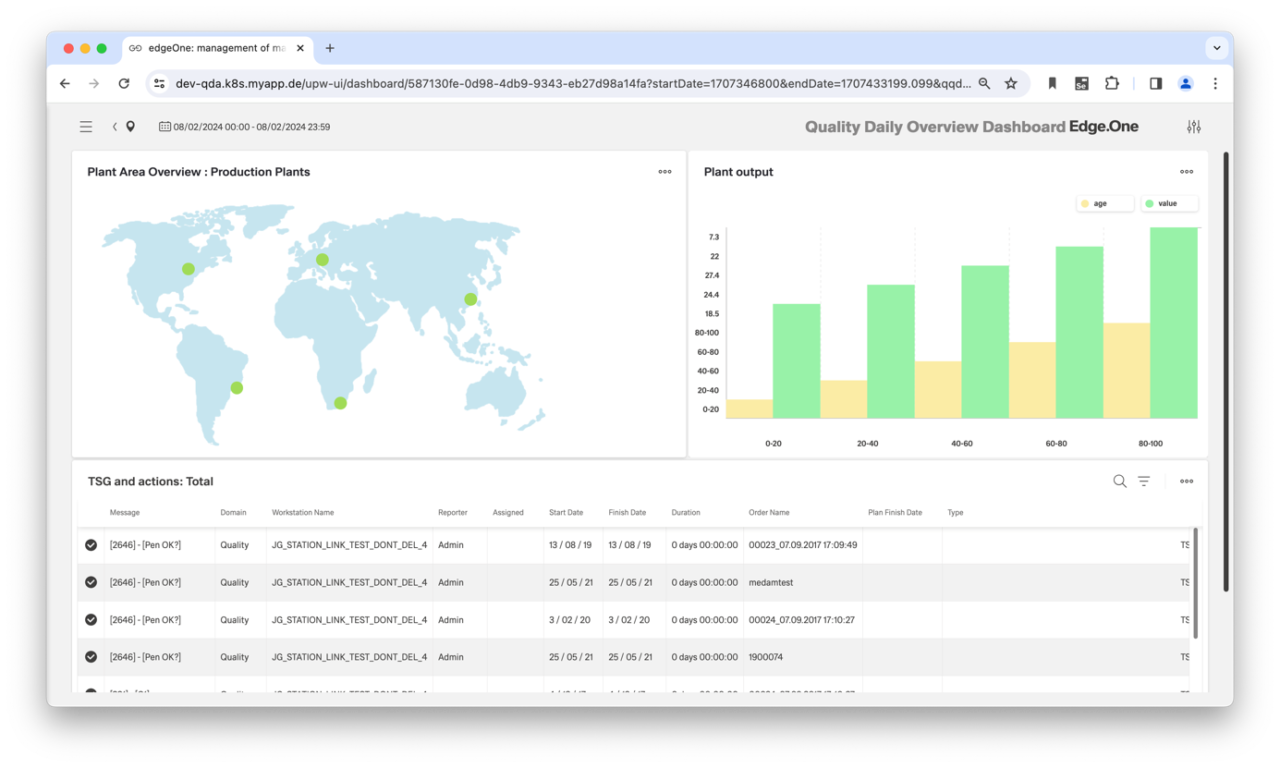
CAQ systems help companies to fulfill their quality assurance (QA) requirements in a standard-compliant and customer-oriented manner. An integral component is a communication and analysis platform that provides the employees involved with the necessary information, planning resources and control tools in a task-oriented manner. The CAQ should also have dashboards that can be used to control, monitor and evaluate ongoing measures across departments. The focus of controlling is primarily on throughput times and quality costs.
What tasks does CAQ software perform?
The CAQ software enables the early detection of quality problems and their cost-optimized elimination. In order to organize and control QM processes in compliance with standards, all IT and OT systems in which QM-related data is generated must be integrated bidirectionally. This enables CAQ software to comply with standards such as ISO 9001:2015 or ISO/TS 16949. This applies along the entire value chain, from development, procurement and production through to delivery and customer service. The continuously acquired QM knowledge is documented in an audit-proof manner and is available across all areas for process improvement.
What should a CAQ system be able to do?
CAQ software should have a modular structure, as the QM requirements can differ significantly depending on the industry, business area, value-added position and size of the company. The modular structure enables lean and agile solutions that adequately map the specific needs of the user companies. The range of required modules begins with the areas of advance quality planning, measurement data management and test equipment management, and extends to statistical process control, laboratory information management and product traceability, as well as the cross-sectional functions of document management and non-conformity management (NCM). If problems do occur, it must be possible to document and analyze them in a clear and structured manner using easy-to-use tools. These include
8D report: a structured problem-solving approach with eight steps that identifies, analyzes and solves complex problems.
Ishikawa diagrams: Also known as fishbone or cause-and-effect diagrams, are graphical representations used to identify the possible causes of a particular problem.
The 5 Whys method: a simple technique in which the question “Why?” is asked repeatedly to identify the underlying causes of a problem.
Who benefits from the use of CAQ software?
No manufacturing company can afford to work without a CAQ system. There is a risk of low productivity, high reject rates, a lack of traceability, manual processes and susceptibility to errors, limited analysis capabilities and non-compliance with norms and standards.
A CAQ system leads to a quality-focused culture in the company. As soon as QM knowledge can be used across departments, all participants in the value chain benefit from it. The most important added values are
- Quality problems can be rectified earlier and more cost-effectively.
- Recalls and withdrawals can be avoided.
- The number and scope of complaints are reduced.
- Cost-intensive reworking is reduced.
- Production waste decreases.
- Quality costs fall.
- The analysis horizon (times and costs) is extended.
Customers benefit from a CAQ system by improving product quality and reliability. Management and executives benefit from a CAQ system because it enables them to plan, monitor and control quality objectives more effectively. They also gain detailed insight into quality performance indicators to make informed decisions. Production and manufacturing: Production and manufacturing departments benefit from a CAQ system as it helps them to monitor manufacturing processes, detect and correct errors at an early stage, improve product quality and reduce waste. And last but not least: Employees benefit from a CAQ system as it helps them to produce high-quality products, make their work more efficient and raise awareness of quality standards within the company.
Get in touch!
Would you like to know more about our solutions? Then please write me using the contact form. I will get back to you as soon as possible.

Dominik Weggler
Sales Team Germanedge